

Whilst serum TSH is regarded clinically as being the most sensitive and specific thyroid function test, in the acute hospital setting, as many as 3% of patients may exhibit TSH concentrations that are <0.1 mU/l (typical TSH reference range 0.3–4.0 mU/l), and in around 75% of these patients, the low TSH can be attributed to the NTIS or the use of glucocorticoid or dopamine medication causing TSH suppression ( Spencer et al. Patients with moderate to severe illness may also show abnormalities in TSH and T 4. In this review, the current state of knowledge regarding these processes will be described together with the current consensus view on the need for clinical intervention with thyroid hormone replacement in such sick patients.Ī decrease in T 3 is the most common finding in ‘euthyroid’ sick patients, occurring in even the mildest forms of non-thyroidal illness (NTI).
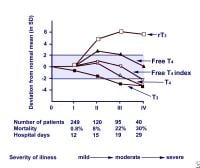
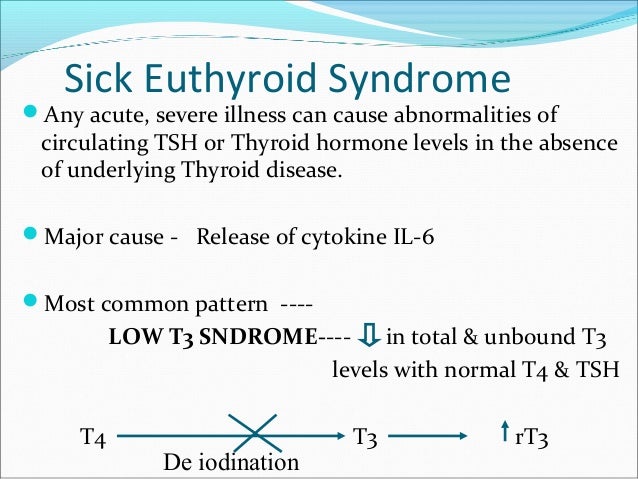
It has been much debated whether these changes in the HPT axis during illness are representative of an associated pathology requiring thyroid hormone replacement therapy or are indeed an adaptive response to ‘stress’ to decrease metabolic rate, which in turn may be beneficial to the sick patient.Ī wide range of mechanisms give rise to the hormonal changes seen in the NTIS these include modifications to the hypothalamic–pituitary axis, altered binding of thyroid hormone to circulating binding proteins, modified entry of thyroid hormone into tissue, changes in thyroid hormone metabolism due to modified expression of the intracellular iodothyronine deiodinases and changes in thyroid hormone receptor (THR) expression or function. ‘Non-thyroidal illness syndrome (NTIS)’ is now more commonly used to describe the typical changes in thyroid-related hormone concentrations that can arise in the serum following any acute or chronic illness that is not caused by an intrinsic abnormality in thyroid function. Sick patients with low serum T 3 are often regarded as being clinically euthyroid, and as a consequence, the alternative term ‘Euthyroid sick syndrome’ was widely used in the past. Changes within the hypothalamic–pituitary–thyroid (HPT) axis also occur in illness and are typically associated with low levels of total triiodothyronine (T 3), and this has given rise to the term ‘low T 3 syndrome’. The extent of this rise is related to the severity of the illness and is critical for survival (Vermes & Beishuizen 2001). The activation of the pituitary–adrenal axis is common, and plasma cortisol concentrations rise rapidly as a result of the acute stress response. Illness may induce profound changes in a number of neuroendocrine systems. In contrast, human and animal models of sepsis and trauma indicate that expression of THRs and their coactivators are decreased in acute illness.
In man, chronic illness leads to an upregulation of thyroid hormone receptor (THR) expression at least in liver and renal failure.

Thyroid hormone transporter expression is up-regulated in many models of the NTIS, thus if diminished tissue uptake of hormone occurs in vivo, it is likely to be the result of impaired transporter function caused by diminished intracellular ATP or plasma inhibitors of transporter action.
#Euthyroid sick syndrome treatment free#
When measured by reliable methods, changes in serum free T 4 and free T 3 are modest in comparison to the fall seen in total thyroid hormone. The decline in serum T 3 and T 4 in models of acute illness precedes the fall in hepatic D1, suggesting that much of the initial fall in these hormones may be attributable to an acute phase response giving rise to a reduction in the thyroid hormone binding capacity of plasma. Data from D1 and D2 knockout mice suggest that these enzymes may have little contribution to the low serum T 3 found in acute illness. This can be signalled by a decrease in leptin caused by malnutrition and possibly a localised increase in hypothalamic T 3 catalyzed by altered expression of hypothalamic iodothyronine deiodinases D2 and D3. Induction of a central hypothyroidism occurs due to a diminution in hypothalamic thyrotropin-releasing hormone. The mechanisms behind the changes in serum triiodothyronine (T 3), thyroxine (T 4) and TSH that occur in the non-thyroidal illness syndrome (NTIS) are becoming clearer.


 0 kommentar(er)
0 kommentar(er)
